Miach Orthopaedics in Westborough has secured $40 million in financing for its U.S. rollout of an implant to improve ACL tear recovery.
The financing comes from $30 million in Series B equity from lead investors Canada-based Sectoral Asset Management and Switzerland-based Endeavor Vision. The remaining $10 million came from the company’s signing of a venture debt term sheet with Santa Clara, Calif.-based Silicon Valley Bank, according to a Wednesday press release from the company.
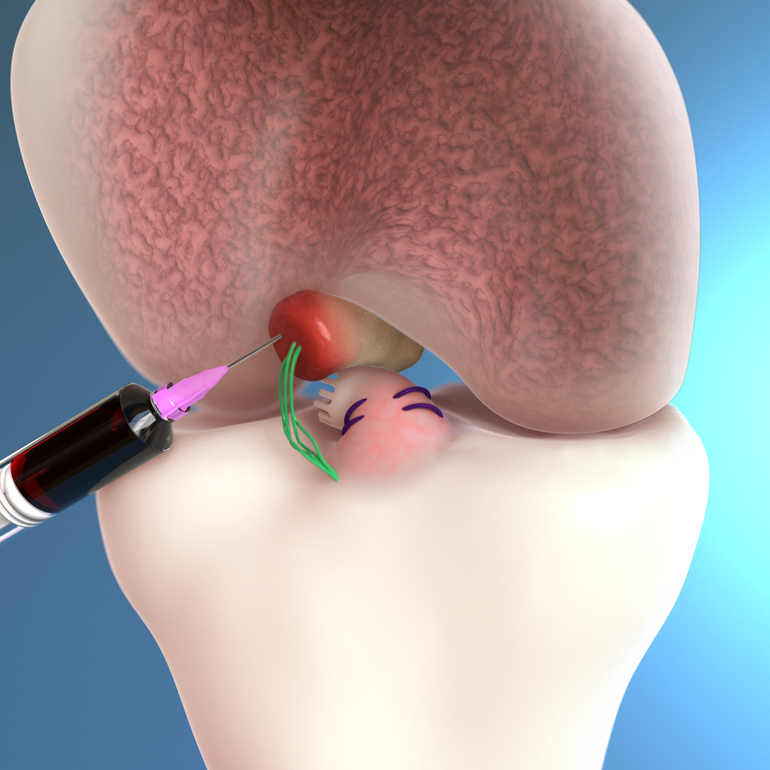
Called the Bridge-Enhanced ACL Restoration Implant, the company’s treatment is intended to shift the way ACL tear treatment is approached by moving from reconstruction to restoration. The implant enables healing of the torn ligament and is a new shift in the standard of care for ACL injuries.
“Since launching our BEAR Implant in fall 2021, we’ve received tremendous interest from both surgeons and patients alike, who understand the benefits of healing a torn ACL rather than replacing it,” Patrick McBrayer, president and CEO of Miach Orthopaedics, said in the press release.

“More than 500 patients have been treated with the BEAR Implant commercially. This additional investment will allow us to expand our presence nationwide and bring the benefits of ACL restoration to more patients with ACL tears.”
The BEAR implant is Miach Orthopaedics’ first product focus and received approval from the U.S. Food and Drug Administration in December 2020.