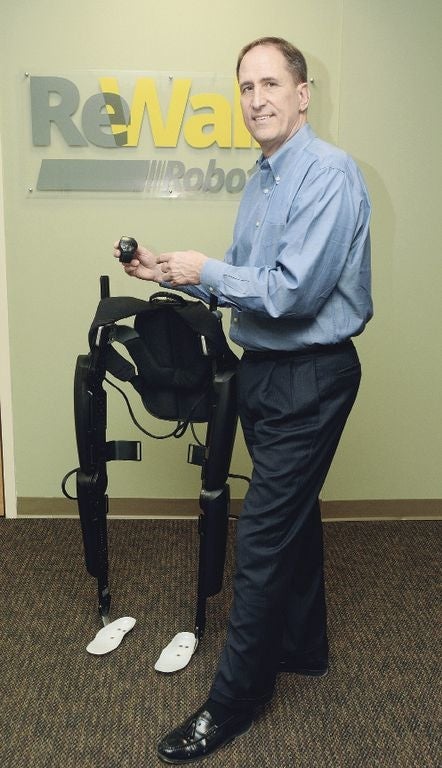
Marlborough exoskelton developer ReWalk Robotics on Tuesday announced it has teamed with the Stanford University School of Medicine to perform a regulator-mandated post-market study of its products.
The study is required by the U.S. Food and Drug Administration on products that could cause significant health risks to the user if they break down. ReWalk first received FDA approval for its exoskelton in 2014.
Let by Stanford, the post-market study will follow 60 patients for one year and could involve as many as 15 centers across the country. California data company Oracle will oversee the data management of the study.