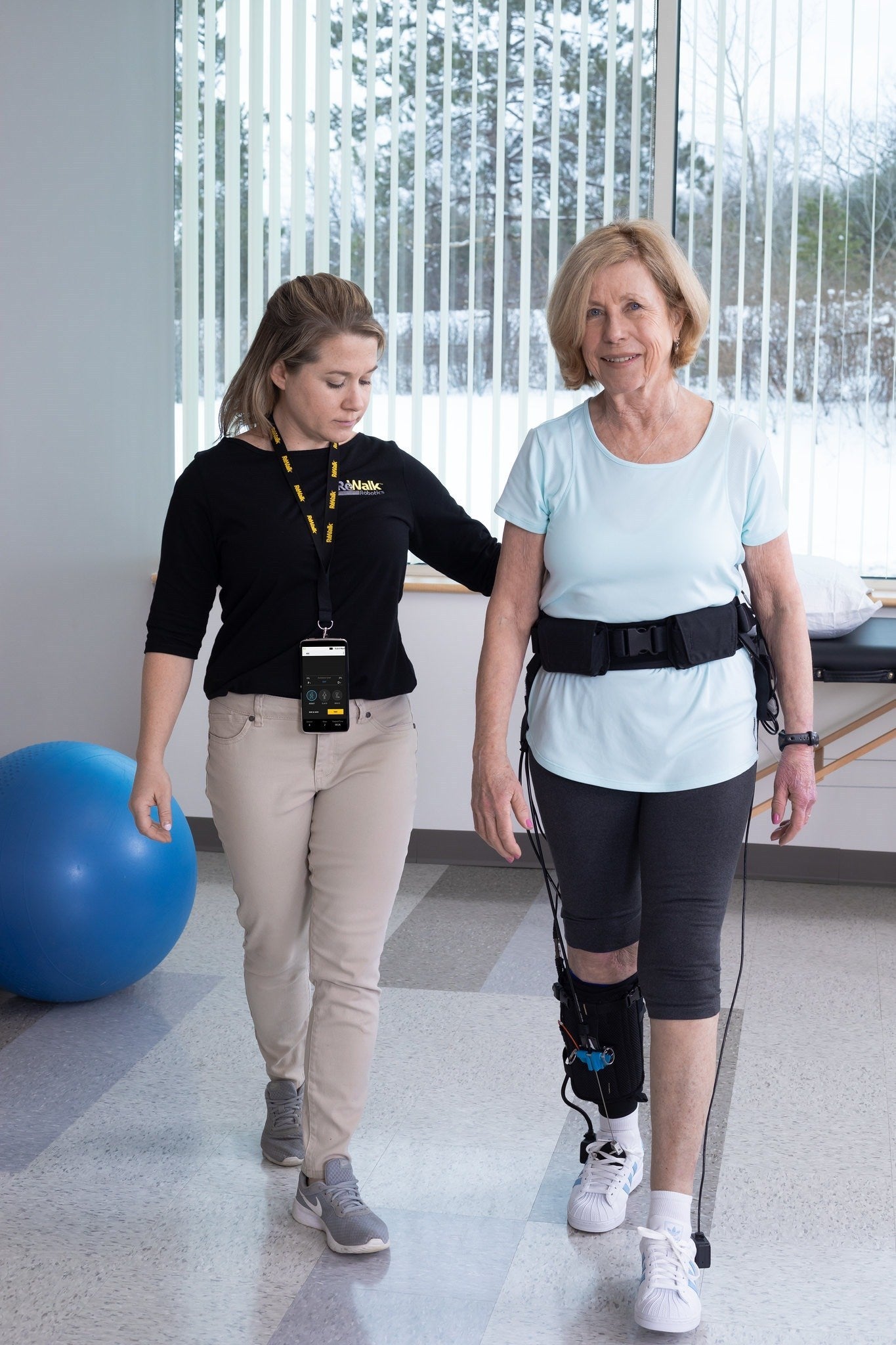
Marlborough biotech manufacturer ReWalk Robotics’ stock skyrocketed Wednesday, rising more than 180% after the company announced late Tuesday the U.S. Food and Drug Administration’s clearance of one of ReWalk’s main products.
The ReStore Exo-Suit, a robotic system designed to help stroke patients regain mobility in physical therapy settings, is now cleared for the U.S. market.
The stock, closing at $3.32 Tuesday, was trading as high as $9.30 Wednesday morning.
In a press release, ReWalk calls the suit the only soft exo-suit to be cleared in the U.S. CEO Larry Jasinski said in a statement the system will open at a cost of $28,900.
“The exo-suit achieves our commercial goal to offer a functional and affordable system that can be utilized in the Main Street clinics in every community,” Jasinski said.
In the announcement and in previous interviews with WBJ, Jasinski has said the system will help the company break even and eventually turn a profit.
The approval comes less than a week after the same product received the CE Mark for clearance in Europe.
According to the company, the suit was originally developed at Harvard University’s Wyss Institute for Biologically Inspired Engineering where it underwent clinical testing demonstrating an ability to improve walking for stroke patients.
The suit is the less costly of the company’s two main products, the other being the ReWalk suit, which helps patients with spinal cord injuries walk again.
That suit is about $100,000, and a majority of those systems are placed with the U.S. Department of Veterans Affairs and via a select few health insurers.
On Wednesday, the company announced an agreement with several accredited investors to purchase more than 1.4 million shares at a price of $7.50 to raise more than $10.8 million.
The company will use those funds for sales marketing and reimbursement expenses for market development for the Restore device. The company will also broaden third-party payor coverage for the ReWalk Personal device for spinal cord injury patients.
ReWalk will also continue the research and development of other applications of the technology for various lower limb disabilities.

