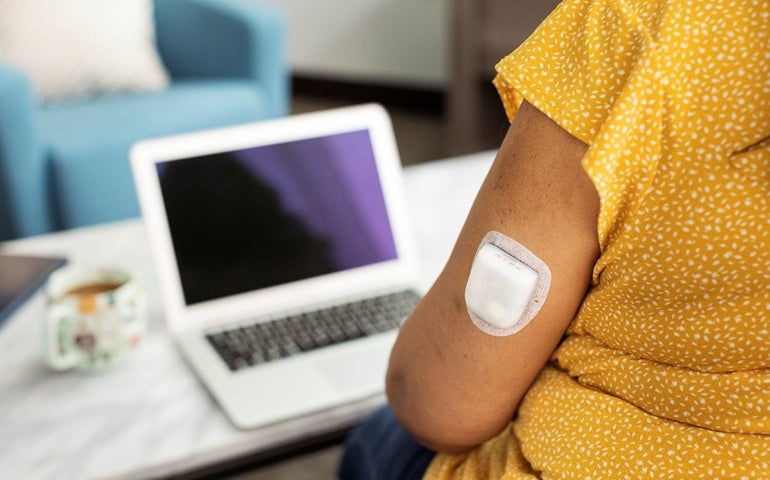
Insulet, an Acton-based medical device firm specializing in diabetes treatment, has launched commercialization efforts for its tubeless insulin device in the United Kingdom.
The tubeless insulin delivery system, called the Omnipod, prevents users from needing to administer multiple daily injections. It will be available in the U.K. for those with type 1 diabetes who are over the age of 2, according to a Tuesday press release from Insulet.
The Omnipod was cleared by the U.S. Food and Drug Administration for use in the United States for the same population in August. Insulet is working with the National Health Service in the U.K. as it rolls out the release of the Omnipod overseas.
“Since its commercial launch in the United States, Omnipod 5 has had a remarkable impact on people with diabetes,” Jim Hollingshead, Insulet president and CEO, said in the press release. “Every day, we hear how this revolutionary experience of Omnipod 5 reduces the burden and improves quality of life for people with diabetes, and we are excited to make a difference in other parts of the world, beginning with the UK. We are committed to making Omnipod 5 an option for as many people as possible, as quickly as we can, and working hard to secure broad access globally.”
Insulet plans to launch the Omnipod in Germany in the fall, according to the press release.
In February, Insulet announced a pair of $25-million acquisitions, doubling its intellectual property.