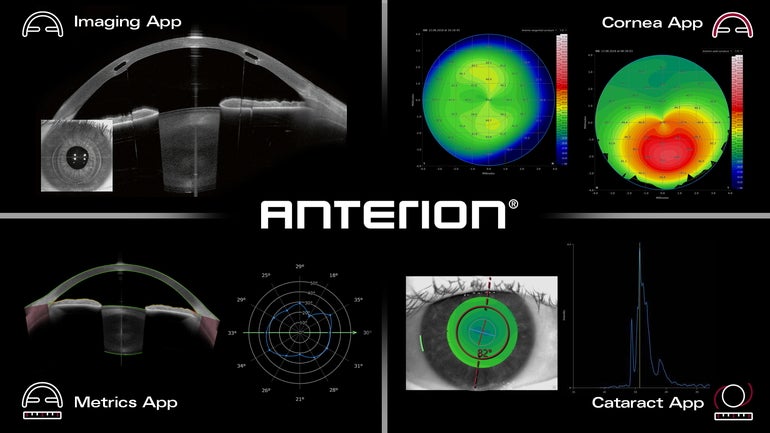
Franklin-based Heidelberg Engineering has received U.S. Food and Drug Administration clearance for its ANTERION platform, which aims to improve eye-imaging diagnostics.
“We believe this device complements our current portfolio of products and arms anterior segment focused practices with the comprehensive data needed to support diagnostic decisions that ultimately improve patient care,” said managing director of Heidelberg Engineering Arianna Schoess Vargas in a Friday press release.
The platform includes eye-tracking technology, the ability to perform anterior segment examinations targeting hard-to-see parts of the eye, and key measurement capabilities.
“Its multifaceted utility, high resolution, rapid image acquisition, and intuitive user interface will make this device an invaluable tool for clinical practice,” said clinical trial principal investigator Dr. Mitchell Dul in the press release.
The platform builds upon the already-cleared imaging app from Heidelberg which assists eye doctors in diagnostics, according to the release.