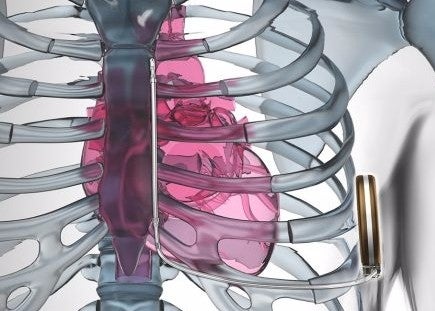
The U.S. Food and Drug Administration (FDA) has approved an implanted defibrillator system from Boston Scientific for use with MRI machines.
The company’s EMBLEM S-ICD systems can now undergo an MRI with the defibrillator implanted, according to the approval. The system leaves the heart and vasculature untouched, thus having benefits over conventional defibrillator leads.
Boston Scientific received CE Mark for the EMBLEM MRI S-ICD System earlier this year and began commercialization in Europe in June. The device will now be offered with MRI labeling.