A device built by Marlborough’s Boston Scientific has just received clearance from the Centers for Medicare and Medicaid Services (CMS) to be used as a non-pharmacological treatment option for stroke risk reduction.
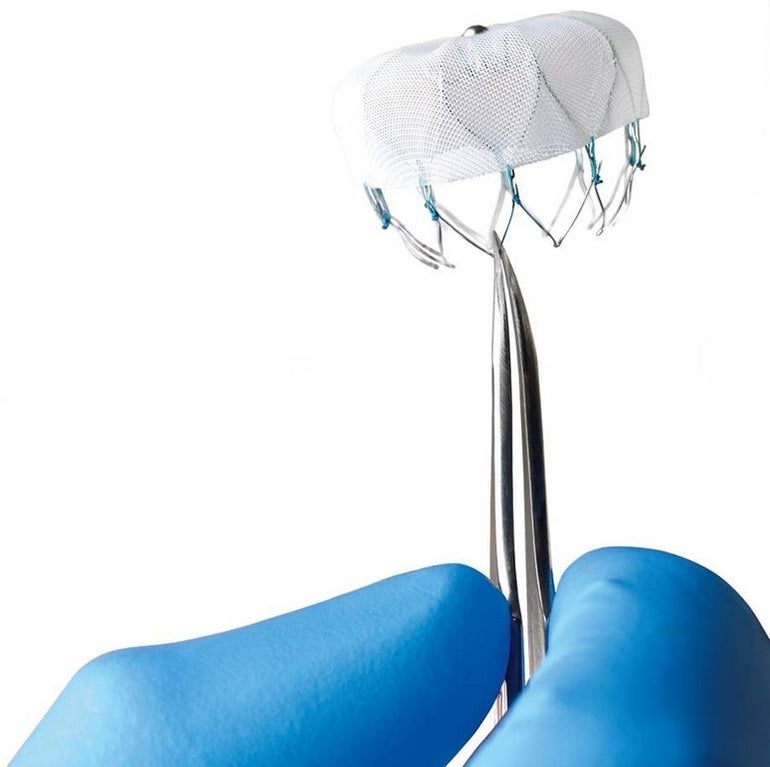
The WATCHMAN LAAC Device is a percutaneous LAAC therapy approved by the U.S. Food and Drug Administration (FDA) in March 2015. It is the only such approved device and now will be able to be accessed by Medicare patients, according to Boston Scientific President and CEO Mike Mahoney, giving them another option outside of a lifetime of anticoagulation medicine.
The WATCHMAN LAAC Device is a catheter-delivered heart implant designed to close the left atrial appendage (LAA) in order to prevent the migration of blood clots from the LAA, thus reducing the incidence of stroke and systemic embolism. The LAA is believed to be the source of more than 90 percent of stroke-causing clots in patients with non-valvular atrial fibrillation, according to Boston Scientific.
Medicare beneficiaries account for the overwhelming majority of patients deemed candidates for the WATCHMAN Device, according to Boston Scientific. The remaining population is represented by private payers. Prior to the CMS final decision, a number of private payers, including several Blue Cross Blue Shield plans, have updated their policies to now cover the WATCHMAN Device.