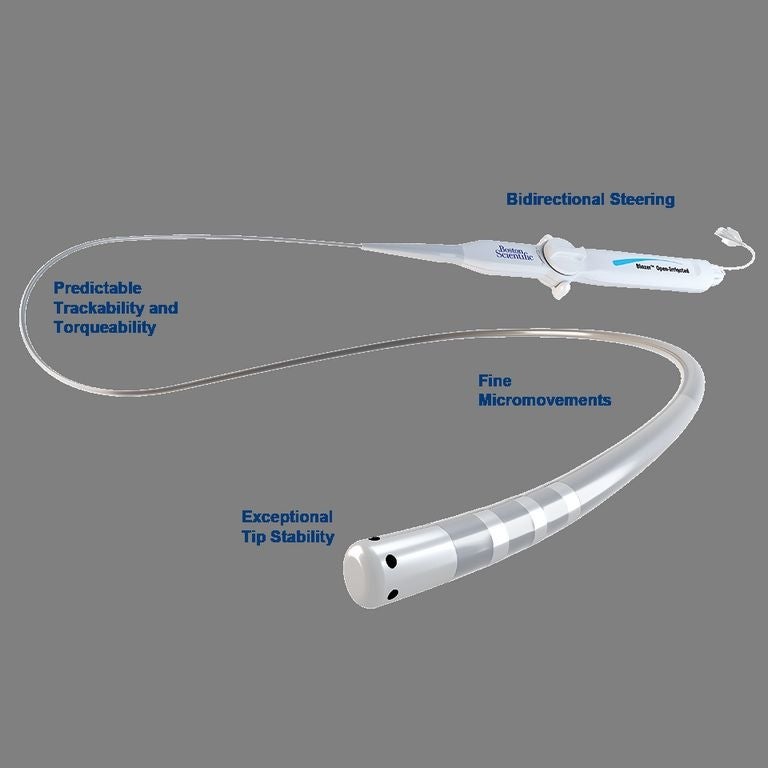
Boston Scientific has received approval from the U.S. Food and Drug Administration for a new catheter designed to treat an abnormal rhythm of the upper chambers of the heart. The approval marks the first time the company will offer an open-irrigated catheter in the U.S. market.
The Blazer Open-Irrigated catheter from the Marlborough company is designed to be used in procedures to restore a normal heart rhythm for patients in atrial flutter. During the procedure, localized electrical energy is delivered through an electrode on the tip of a catheter into the heart muscle, creating heat to destroy a small area of the tissue responsible for the abnormal heart rhythm. The Blazer catheter includes technology intended to cool the catheter tip consistently during the procedure and thus improve the quality of the lesion.
The approval stems from a trial that included 302 patients at 24 sites in the U.S. and follows the January approval of the IntellaTip MiFi catheter for use in all cardiac ablation procedures in Europe. The two approvals represent a continued focus for the company on electrophysiology, according to Kenneth Stein, M.D., chief medical officer of Cardiac Rhythm Management at Boston Scientific.