Boston Scientific receives FDA approval for opioid-alternate back pain treatment
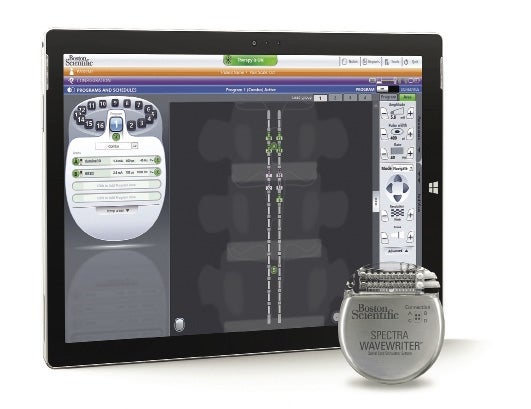 Image | Courtesy of Boston Scientific
The Spectra WaveWriter Spinal Cord Stimulator System combats pain without opioids.
Image | Courtesy of Boston Scientific
The Spectra WaveWriter Spinal Cord Stimulator System combats pain without opioids.
Marlborough medical technology firm Boston Scientific has received approval from the U.S. Food and Drug Administration for its WaveWriter Alpha spinal cord stimulator device to be used to treat low back and leg pain deriving from a condition referred to as non-surgical back pain.
The device, which received FDA approval to be used to treat chronic intractable pain of the trunk and/or limbs in December 2020, utilizes mild electrical currents to interrupt pain signals traveling to the brain, opening the door to effective pain management without the use of opioids.
First-line treatment of chronic back pain has traditionally relied on medical management via physical therapy and medication, which is not effective for many people, according to a press release issued by Boston Scientific on Tuesday.
″Early and effective intervention with SCS therapy is associated with long-term success and improved outcomes for people living with chronic back pain,″ Jim Cassidy, president for neuromodulation at Boston Scientific, said in the press release. ″Today’s approval, combined with the recent indication for diabetic peripheral neuropathy, extends the reach of our robust portfolio to help physicians deliver individualized care across a wide spectrum of lower back pain issues.″
FDA approval was achieved following a one-year randomized trial which found 84% of patients treated with the WaveWriter system saw significant pain relief, with more than 50% of participants seeing sustained improvement in their ability to participate in activities of daily living.
WaveWriter utilizes four Bluetooth-enabled implantable pulse generators to send mild electric pulses to the spinal cord without the tingling sensations typically associated with paresthesia-based therapy. Boston Scientific began selling the device in the U.S. market in January 2021.












0 Comments